Research Highlights for Epigenetics
Retina Epigenetic Landscape
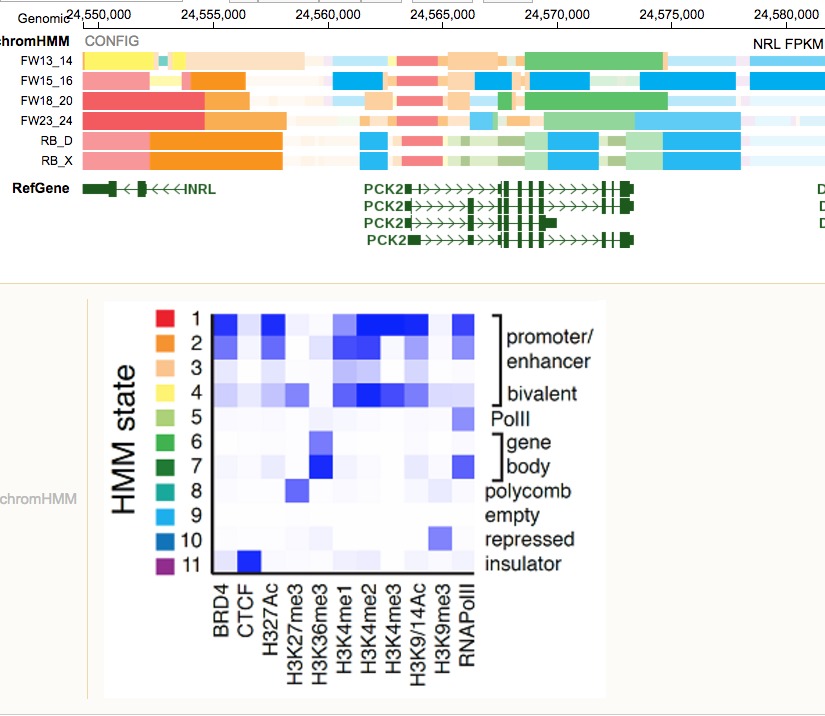
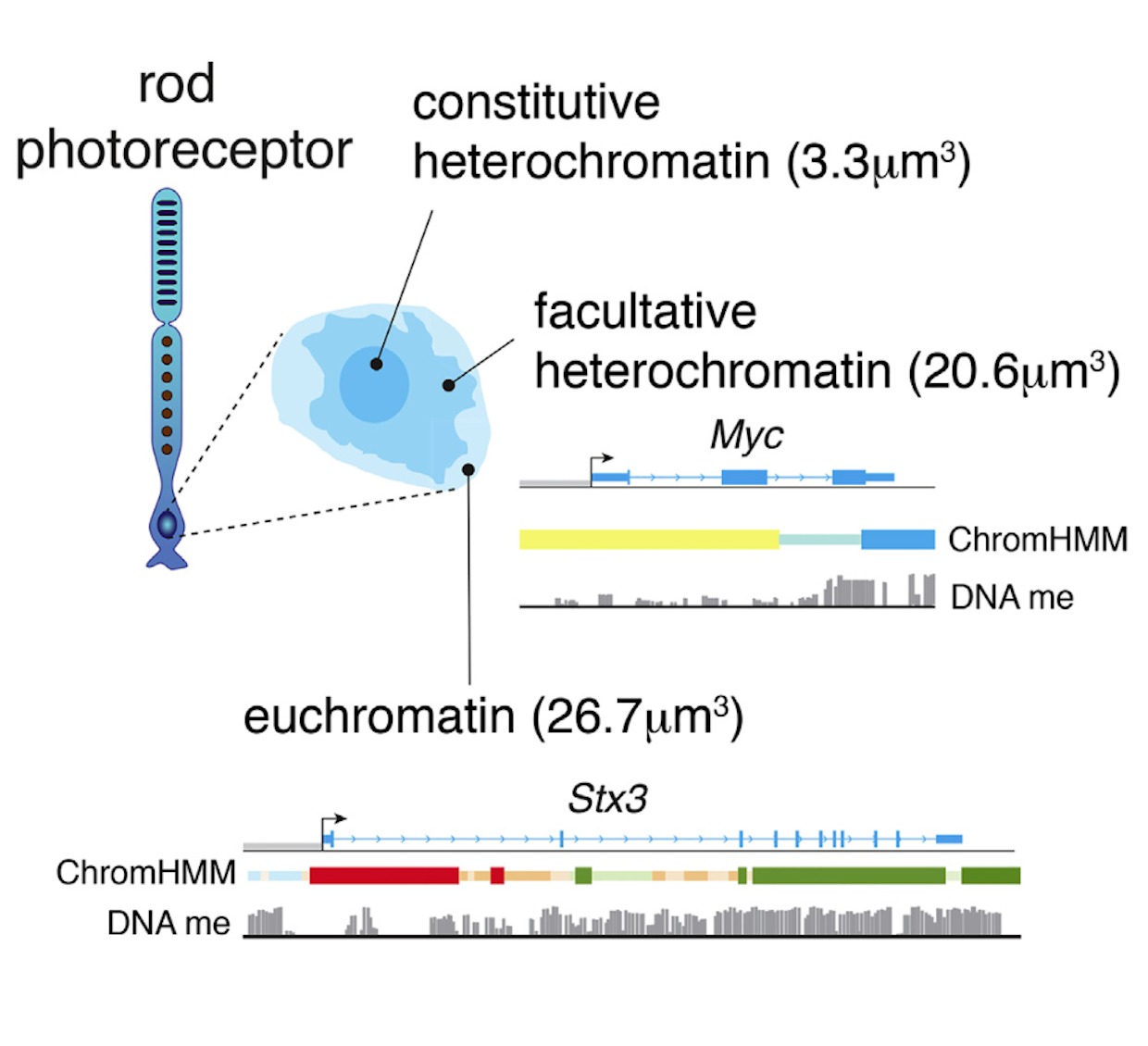
In the developing retina, multipotent neural progenitors undergo unidirectional differentiation in a precise spatiotemporal order. Here we profile the epigenetic and transcriptional changes that occur during retinogenesis in mice and humans. Although some progenitor genes and cell cycle genes were epigenetically silenced during retinogenesis, the most dramatic change was derepression of cell-type-specific differentiation programs. We identified developmental-stage-specific super-enhancers and showed that most epigenetic changes are conserved in humans and mice. To determine how the epigenome changes during tumorigenesis and reprogramming, we performed integrated epigenetic analysis of murine and human retinoblastomas and induced pluripotent stem cells (iPSCs) derived from murine rod photoreceptors. The retinoblastoma epigenome mapped to the developmental stage when retinal progenitors switch from neurogenic to terminal patterns of cell division. The epigenome of retinoblastomas was more similar to that of the normal retina than that of retina-derived iPSCs, and we identified retina-specific epigenetic memory.
- We first profiled the epigentics landscape for 8 mice, 4 human development stages and 1 mouse, 1 human retinoblastoma cell line. Neuron, 2017
- Then we systematically profiles epigentics landscape for iPSC and find the key epigenetical factors prevent iPSC successful developed into retina. Cell Stem Cell, 2015, Cell Report, 2018
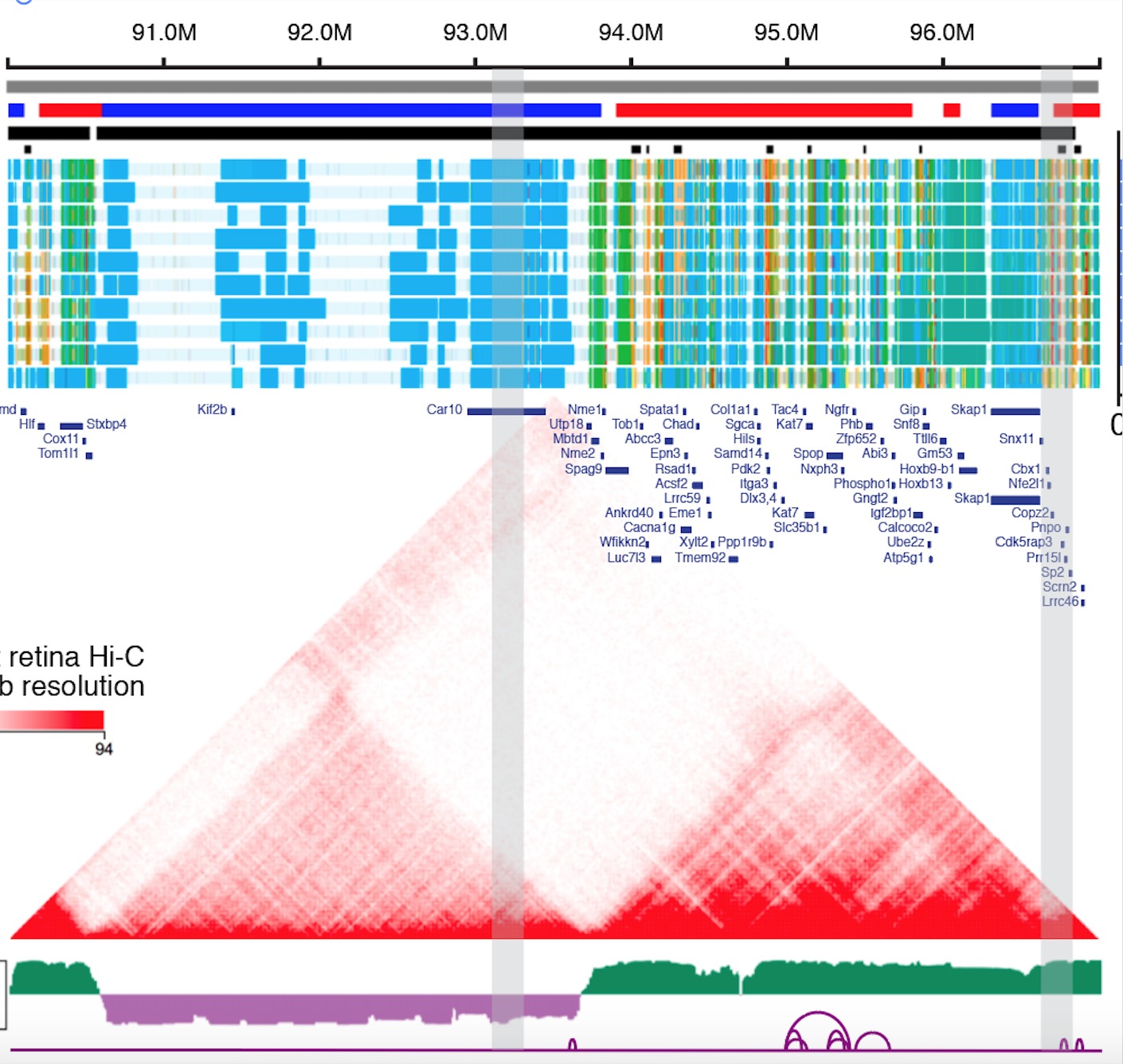
- Recently, we also intergrated 3D Genome profiles and validated a Vsx2 Super-Enhancer could affect the bipolar cell Neuron, 2019
Their profiles could be access online at
Epigenetics Regulators
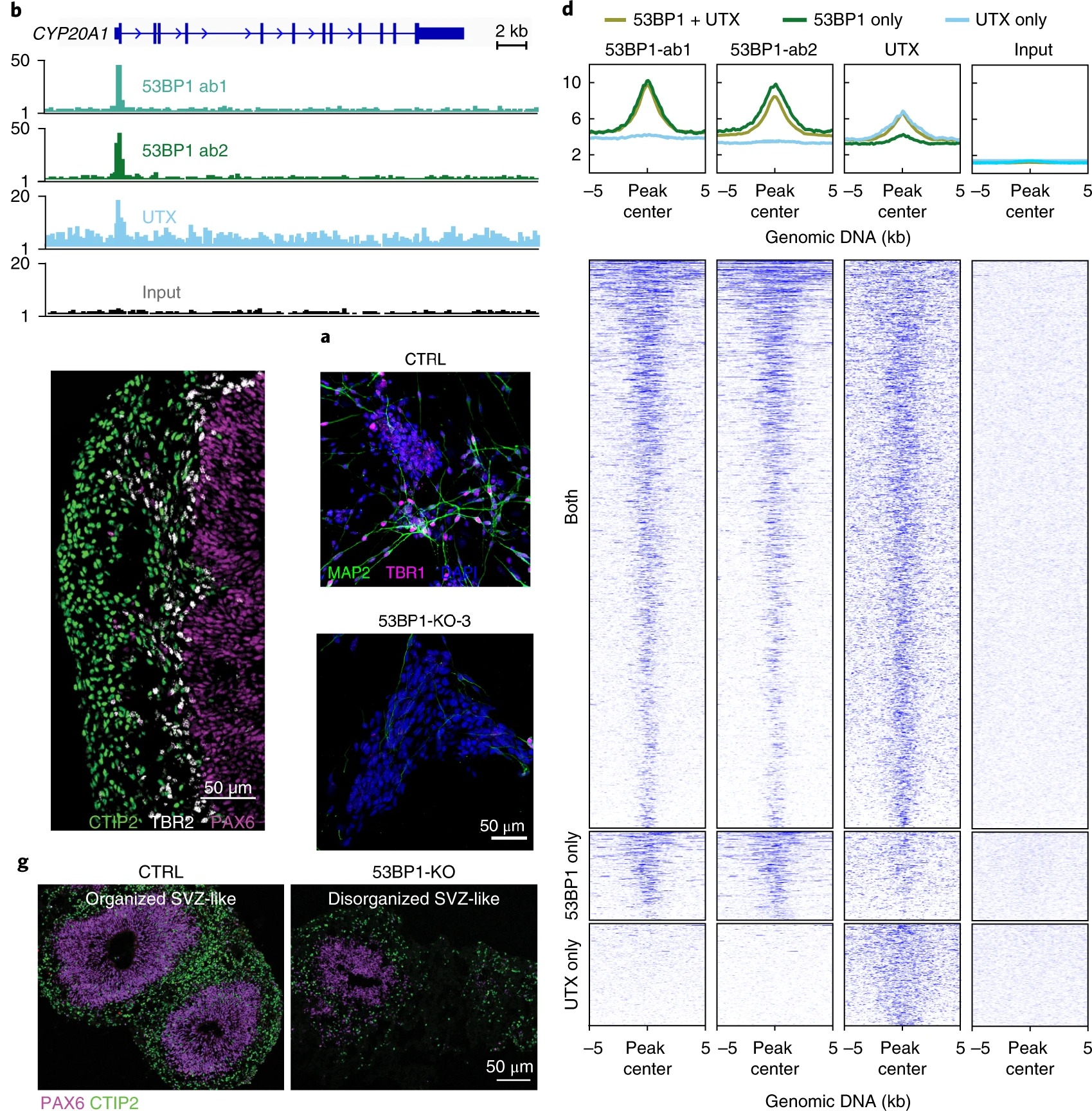
UTX/KDM6A is a chromatin modifier required for development and neural lineage specification, but how it controls these biological processes is unclear. To determine the molecular mechanisms of UTX:
- We first found UTX and 53BP1 directly interact and co-occupy promoters in human embryonic stem cells and differentiating neural progenitor cells. Human 53BP1 contains a UTX-binding site that diverges from its mouse homolog by 41%, and disruption of the 53BP1–UTX interaction abrogated human, but not mouse, neurogenesis in vitro. The 53BP1–UTX interaction is required to upregulate key neurodevelopmental genes during the differentiation of human embryonic stem cells into neurons or into cortical organoids. Nature Neuroscience, 2019
- Next we found UTX suppresses AP-1 and a gliogenesis program during neural differentiation of human pluripotent stem cells using ChIP-Seq and ATAC-Seq. Epigenetics & Chromatin, 2020
Polycomb repressive complex 2 (PRC2) is another crucial chromatin modifier in executing neurodevelopmental programs:
- We found a novel player Ybx1 that could fine-tunes PRC2 activities to control embryonic brain development. Nature Communication, 2020